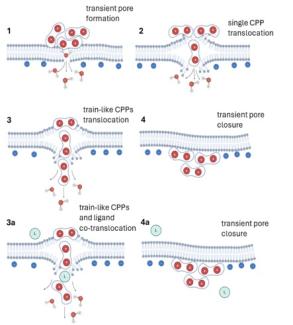
Controlled transport of cell-penetrating peptides across biological membranes
Controlled transport of cargo into cells is vital for life-sustaining. Alongside the active ATP driven process of endocytosis, which plays a pivotal role in the transport of cargo across membranes, there is another promising method known as direct passive energy-independent translocation, which is particularly relevant for controlled drug delivery. Short, positively charged cell-penetrating peptides (CPPs) are commonly utilized vectors for this purpose. In this new project, we want to explore the mechanism of passive translocation of CPPs at the molecular level through advanced single-molecule fluorescence experiments and molecular simulations (Dr. Mario Vazdar and Prof. Pavel Jungwirth). Our focus is on understanding crucial steps in this process, including adsorption, aggregation, and translocation across advanced model membrane systems. We believe that insights gained from this investigation will lay the groundwork for designing smarter drug delivery systems by identifying crucial molecular interactions during the transport of matter across biological membranes.
Selected publications
- Nguyen, M. T. H.; Biriukov, D.; Tempra, C.; Baxova, K.; Martinez-Seara, H.; Evci, H.; Singh, V.; Šachl, R.; Hof, M.; Jungwirth, P.; Javanainen, M.; Vazdar, M. Ionic Strength and Solution Composition Dictate the Adsorption of Cell-Penetrating Peptides onto Phosphatidylcholine Membranes. Langmuir 2022, 38 (37) , pp.11284-11295
- Allolio, C., Magarkar, A., Jurkiewicz, J., Baxová, K., Javanainen, M., Mason, P.E., Šachl, R., Cebecauer, M., Hof, M., Horinek, D., Heinz, V., Rachel, R., Ziegler, C.M., Schröfel, A. and Jungwirth, P. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc. Natl. Acad. Sci., 115, 11923–11928, (2018),