Our scientists' discovery writes new chapter in electrochemistry textbooks enabling development of smart materials
Scientists from the J. Heyrovsky Institute of Physical Chemistry of the Czech Academy of Sciences, in collaboration with the University of Paris, have achieved a breakthrough result. It could influence the future development of smart materials. The discovery, which challenges one of the fundamental principles of electrochemistry concerning electron manipulation, was recently published in the journal Angewandte Chemie.
Scientists create smart materials in various ways. Specifically, electrochemists utilize electron manipulation. This allows them to configure the desired properties of smart materials for diverse technological applications. The J. Heyrovsky Institute of Physical Chemistry of the Czech Academy of Sciences, in collaboration with the University of Paris, has now discovered a new method of multi-electron transfer that requires less energy.
Molecular flexibility changes electrochemical behavior
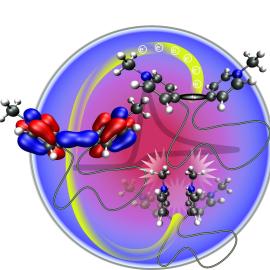
Magdaléna Hromadová's team studies chemical reactions in which molecules accept or give up electrons. In one of their experiments, the scientists focused on a type of molecule consisting of two redox centers interconnected by a non-conductive aliphatic linker composed of repeating methylene units. Redox centers represent sites in the molecule where electron transfer (acceptance or donation) occurs.
Scientists previously assumed that the aliphatic chain blocks communication between these centers in the molecule, thus each redox site would bind its own electron during charge transfer. However, new results have shown that the chain is more flexible than they thought, allowing communication between centers.
"Using quantum-chemical calculations, we demonstrated and experimentally verified that the electrons were not localized on individual redox centers, but were shared in a newly formed orbital. Less energy was required to accept the second electron," explained Magdaléna Hromadová, head of the Department of Electrochemistry at the Nanoscale. "Contrary to expectations, a different molecule was also formed," she added.
A new chapter in electrochemistry textbooks
Scientists had observed two-electron transfers before. They explained the mechanism by formation of radicals which subsequently combine into a chemical bond. However, new results have shown that the previous explanation was often incorrect. In reality, there was no interaction of radicals, but both redox centers shared electrons simultaneously in a qualitatively new way.
"With our article in Angewandte Chemie, we want to call on colleagues in the field of electrochemistry to take this observed phenomenon into account in their research and design of new functional materials," said Magdaléna Hromadová.
The discovery has the potential to influence the creation of new smart materials, such as molecular switches, which are key building blocks of the nanoworld. These switches are used in genetics, medicine, and electronics.
The project received support from OP JAK
The research was conducted as part of the AMULET (Advanced MUltiscaLe materials for key Enabling Technologies) project, which received financial support from the Operational Programme Jan Amos Komenský of the Ministry of Education, Youth and Sports and is co-financed by the European Union funds. The project develops progressive, so-called multiscale materials with extensive application potential - for example in electrical engineering, medicine, or environmental technologies.